Doctors need better ways to figure out fevers in newborns
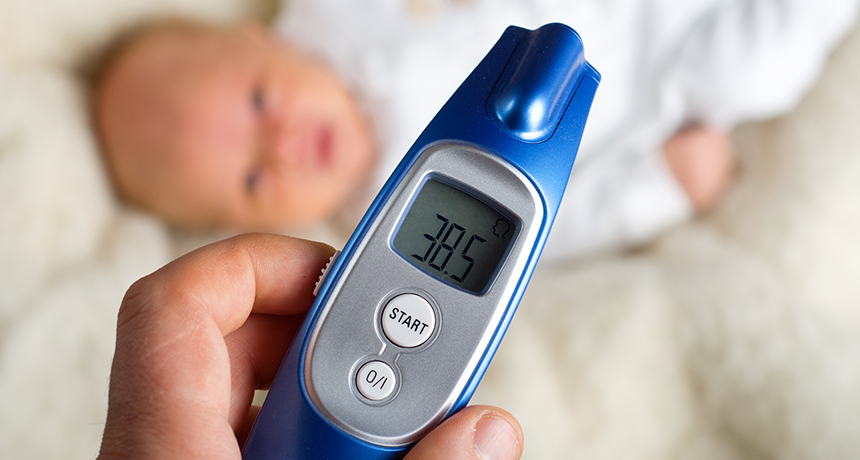
Two days after my first daughter was born, her pediatrician paid a house call to examine her newest patient. After packing up her gear, she told me something alarming: “For the next few months, a fever is an emergency.” If we measured a rectal temperature at or above 100.4° Fahrenheit, go to the hospital, she said. Call her on the way, but don’t wait.
I, of course, had no idea that a fever constituted an emergency. But our pediatrician explained that a fever in a very young infant can signal a fast-moving and dangerous bacterial infection. These infections are rare (and fortunately becoming even rarer thanks to newly created vaccines). But they’re serious, and newborns are particularly susceptible.
I’ve since heard from friends who have been through this emergency. Their newborns were poked, prodded and monitored by anxious doctors, in the hopes of quickly ruling out a serious bacterial infection. For infants younger than two months, it’s “enormously difficult to tell if an infant is seriously ill and requires antibiotics and/or hospitalization,” says Howard Bauchner, a pediatrician formerly at Boston University School of Medicine and now editor in chief of the Journal of the American Medical Association.
A new research approach, described in two JAMA papers published in August, may ultimately lead to better ways to find the cause of a fever.
These days, for most (but not all) very young infants, their arrival at a hospital will trigger a workup that includes a urine culture and a blood draw. Often doctors will perform a lumbar puncture, more commonly known as a spinal tap, to draw a sample of cerebrospinal fluid from the area around the spinal cord.
Doctors collect these fluids to look for bacteria. Blood, urine and cerebrospinal fluid are smeared onto culture dishes, and doctors wait and see if any bacteria grow. In the meantime, the feverish infant may be started on antibiotics, just in case. But this approach has its limitations. Bacterial cultivation can take several days. The antibiotics may not be necessary. And needless to say, it’s not easy to get those fluids, particularly from a newborn.
Some scientists believe that instead of looking for bacteria or viruses directly, we ought to be looking at how our body responds to them. Unfortunately, the symptoms of a bacterial and viral infection are frustratingly similar. “You get a fever. You feel sick,” says computational immunologist Purvesh Khatri of Stanford University. Sadly, there are no obvious telltale symptoms of one or the other, not even green snot. In very young infants, a fever might be the only sign that something is amiss.
But more subtle clues could betray the cause of the fever. When confronted with an infection, our immune systems ramp up in specific ways. Depending on whether we are fighting a viral or bacterial foe, different genes turn up their activity. “The immune system knows what’s going on,” Khatri says. That means that if we could identify the genes that reliably get ramped up by viruses and those that get ramped up by bacteria, then we could categorize the infection based on our genetic response.
That’s the approach used by two groups of researchers, whose study results both appear in the August 23/30 JAMA. One group found that in children younger than 2, two specific genes could help make the call on infection type. Using blood samples, the scientists found that one of the genes ramped up its activity in response to a viral infection, and the other responded to a bacterial infection.
The other study looked at immune responses in even younger children. In infants younger than 60 days, the activity of 66 genes measured in blood samples did a pretty good job of distinguishing between bacterial and viral infections. “These are really exciting preliminary results,” says Khatri, who has used a similar method for adults. “We need to do more work.”
Bauchner points out that in order to be useful, “the test would have to be very, very accurate in very young infants.” There’s very little room for error. “Only time will tell how good these tests will be,” he says. In an editorial that accompanied the two studies, he evoked the promise of these methods. If other experiments replicate and refine the results of these studies, he could envision a day in which the parents of a feverish newborn could do a test at home, call their doctor and together decide if the child needs more care.
That kind of test isn’t here yet, but scientists are working on it. The technology couldn’t come soon enough for doctors and parents desperate to figure out a fever.



